Evolution of mimetic coloration in bumble bees
The ~250 species of the cold-adapted bumble bees are exceptionally color diverse. One of the main factors driving such variation is Müllerian mimicry – taxonomically diverse species in the same geographic region have converged on similar color patterns as selection favors shared advertisement of their toxicity (their sting) to predators. Bumble bee color diversity provides extensive replicates for understanding the genomic targets of evolution (evolutionary genetics) (Hines and Rahman, 2019), how ecological factors drive adaptive phenotypes (evolutionary ecology), and how developmental gene modifications create phenotypic variation (evo-devo).
We are studying how changes in genomes and transcriptomes lead to diverse mimetic phenotypes in these bees. We have discovered key segmentation genes (Hox genes) switch segmental location late in development to cause evolutionary shifts in the location of red segmental stripes (Tian et al., 2019) and that these genes are repeatedly employed with the convergent evolution of mimics. We also have characterized mimicry complexes in the United States and examined the factors promoting their evolution.
 Photo credits: J. Cnaani (upper right), Sam Droege, USGS Survey (upper middle)
Photo credits: J. Cnaani (upper right), Sam Droege, USGS Survey (upper middle)
Bumble bee conservation
Bumble bee diversity has changed dramatically in the last 30 years due to a combination of habitat loss, pesticides, pathogens, and climate change. We are studying several of the major factors threatening these bees. We are currently studying:
- Habitat needs and resilience to anthropogenic change across bumble bee species and the physiological factors driving these preferences.
- Myriad factors in bumble bees impacting thermal tolerance.
- The environmental factors driving pathogen loads in wild bees. Using landscape modeling, we are examining the landscape factors, such as habitat, climate, and disturbance, that most impact bumble bee pathogen loads, and are examining how pathogens fluctuate across time and by bee species.
Mechanisms of gall induction by gall wasps
Gall wasps oviposit into developing plant tissues where larvae induce the formation of elaborate and predictable gall morphologies. The ways in which both adults and developing larvae induce this developmental change remain largely unknown. With an NSF funded grant, we are combining comparative phylogenomics, comparative gland morphology, and transcriptomic and metabolomic approaches to explore potential mechanisms of gall induction. These analyses are comparing across species, sexual/asexual generations, and larval/adult generations of gall wasps.

Just a sampling of some of the incredible diversity of gall morphologies induced by gall wasp species. From Left: Wooly catkin gall wasp (Callyrhytis quercusoperator) including both asexual and sexual galling generations, Hedgehog gall (Acraspis erinacei), Honeycomb leaf gall (Callyrhytis favosa), Clustered midrib gall (Andricus dimorphus), Wooly oak gall (Callyrhytis lanata). Images by collaborator Andy Deans, available on iNaturalist.
Role of nectar chemistry in bumble bee forage preferences and health
We are examining the role of plant defense compounds in nectar, focusing on milkweed toxins (cardenolides), in bumble bee visitation bias (Villalona et al., 2020), the role of pollen nutrient ratios in driving bumble bee preferences, and how secondary compounds in nectar impact ability to tolerate stressors.
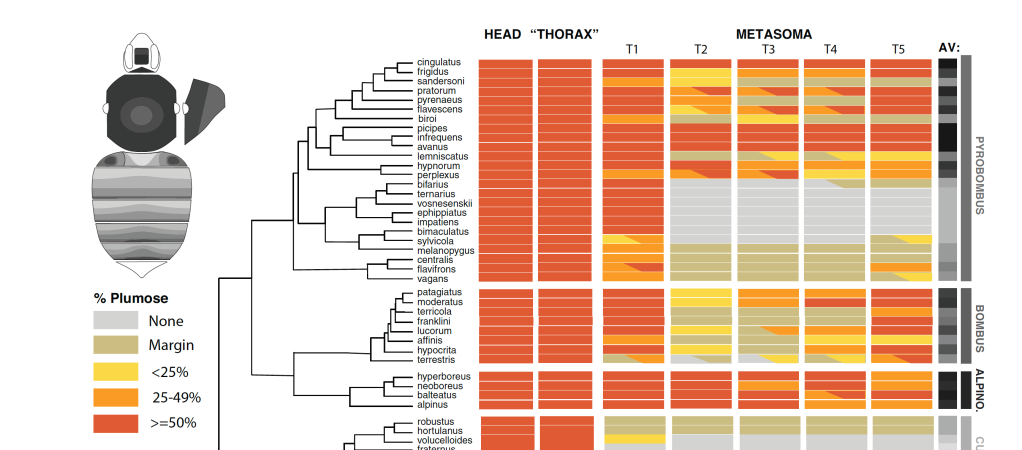
Bumble bee systematics, evolution, and natural history
We have several projects examining phylogenetics, population genetics/species delimitation, biogeography, and the evolution of life history traits in the bumble bees. (e.g., setal diversity below, Hines et al., 2022).